STOMACH INJURY SETTLEMENTS
OZEMPIC, WEGOVY, and RYBELUS LAWSUIT INFORMATION
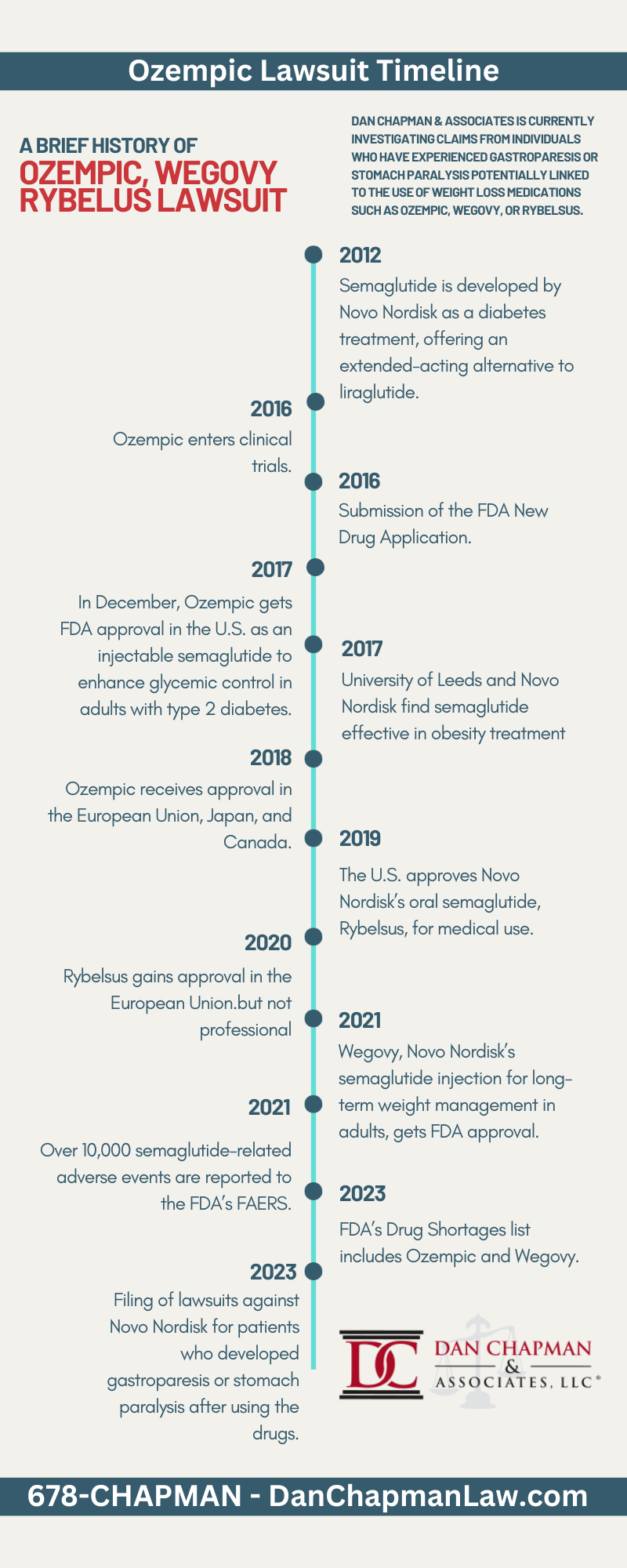
Dan Chapman & Associates is currently investigating claims from individuals who have experienced gastroparesis or stomach paralysis, or bowel blockages potentially linked to the use of weight loss medications such as Ozempic, Wegovy, or Rybelsus. This inquiry stems from concerns that the manufacturer may not have provided sufficient warnings about these serious gastrointestinal side effects to patients and healthcare professionals.
With a long-standing reputation in pursuing legal actions against pharmaceutical companies, Dan Chapman & Associates has been a formidable presence in this field since 1955. Recognized in prestigious listings such as Best Lawyers in America and The National Trial Lawyers Hall of Fame, our firm is well-equipped to manage your Ozempic, Wegovy, or Rybelsus lawsuit related to gastroparesis or stomach paralysis.
Contact us today if you have suffered from any of the following:
- Medical treatment costs (past and future), including surgery
- Medical expenses (past and future)
- Lost income (past and future)
- Pain and suffering related to injuries, treatment, and recovery (past and future)
- Loss of enjoyment of life (past and future)
- Loss of earning capacity
- Possible punitive damages
Our legal team is dedicated to securing the highest possible financial restitution for the hardships you’ve endured due to gastroparesis or stomach paralysis, following the use of diabetes weight loss medications like Ozempic, Wegovy, or Rybelsus.
Is It Possible to Pursue Legal Action for Gastroparesis or Stomach Paralysis Caused by Ozempic?
Our legal team is actively examining cases for people who were treated with GLP-1 RA therapies under the Ozempic, Rybelsus, or Wegovy brands (either in pill form or through injections) and meet the following criteria:
- Are aged 75 or younger; and
- Were diagnosed with gastroparesis, stomach paralysis, or gastric obstruction during or within 30 days after ceasing the use of these medications; and
- Needed to visit the emergency room or were admitted to a hospital.
What Are the Uses of Ozempic, Wegovy, and Rybelsus?
Ozempic, Wegovy, and Rybelsus are part of the glucagon-like peptide-1 receptor agonists (GLP-1 RA) drug class, all containing the same primary active component, semaglutide.
Understanding Semaglutide
As a GLP-1 Receptor Agonist Analog-Type medication, semaglutide is prescribed for managing high blood sugar in those with type 2 diabetes mellitus. This drug works by imitating a natural human glucagon-like peptide (GLP-1), which helps in slowing down food transit through the stomach.
Distinguishing Between These Weight-Loss Medications
Ozempic and Wegovy are both administered through subcutaneous injections. The main distinction lies in their intended use and dosage: Ozempic targets type 2 diabetes management with weekly doses ranging from 0.5 to 1 mg, whereas Wegovy, at a weekly dose of 2.4 mg, is FDA-approved specifically for weight loss. Rybelsus, similar to Ozempic in treating type 2 diabetes mellitus, differs in its administration as a daily oral tablet.
What Are the Key Legal Considerations Surrounding Gastroparesis or Stomach Paralysis Related to Semaglutide Medications?
The central legal concern may revolve around whether Novo Nordisk, the maker of Ozempic, Wegovy, and Rybelsus, was aware or ought to have been aware of the increased risk of gastroparesis or stomach paralysis associated with these drugs, yet neglected to adequately inform doctors and patients about this danger.
What Exactly is Gastroparesis or Stomach Paralysis?
Gastroparesis refers to a disorder where the stomach fails to empty its contents effectively. Mayo Clinic notes that in a normal functioning stomach, robust muscles contract to propel food along the digestive system. But in someone with gastroparesis, this motility is significantly reduced or, in some instances, completely non-functional.
Consequently, food remains in the stomach for an extended period instead of progressing to the small intestine for digestion, as it either doesn’t empty or doesn’t empty as thoroughly as it should.
How Frequently Does Gastroparesis or Stomach Paralysis Occur?
A 2009 research published in the Gastroenterology scientific journal indicated that gastroparesis is relatively rare, affecting just one in every 100,000 individuals (Jung HK, Choung RS, Locke GR III, et al. “The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006.” Gastroenterology. 2009;136(4):1225–1233.).
What Are the Indicators and Symptoms Associated with Gastroparesis or Stomach Paralysis?
Gastroparesis can adversely affect a patient’s blood sugar regulation and nutritional intake, as well as hinder proper digestion. Symptoms associated with this condition encompass:
- Nausea
- Vomiting
- Abdominal bloating
- Abdominal discomfort
- Sensation of fullness after consuming minimal food
- Changes in blood sugar levels
- Acid reflux
- Nutritional deficiencies
- Unintentional weight loss
- Decreased appetite
What Are the Available Treatments for Gastroparesis or Stomach Paralysis?
Currently, there is no known cure for gastroparesis. Treatment options available primarily provide temporary relief, as noted by MedlinePlus, a reputable resource compiling health information from the National Library of Medicine (NLM), the National Institutes of Health (NIH), and various other governmental and health-focused entities.
Complications Arising from Gastroparesis or Stomach Paralysis
According to the National Institute of Diabetes and Digestive and Kidney Diseases, gastroparesis can lead to various complications, such as:
- Dehydration due to persistent vomiting
- Malnutrition due to inadequate absorption of nutrients
- Challenges in managing blood sugar levels
- Inadequate calorie consumption
- Formation of bezoars (solid masses of undigested food in the stomach) which can result in:
- Obstruction
- Ulcers
- Internal bleeding
- Reduced quality of life
In severe cases, if a bezoar prevents food from passing into the small intestine, gastroparesis may become a life-threatening condition.
.
Key Timeline Details in the Ozempic, Wegovy, and Rybelsus Legal Cases
- 2012: Semaglutide is developed by Novo Nordisk as a diabetes treatment, offering an extended-acting alternative to liraglutide.
- 2016: Ozempic enters clinical trials.
- 2016: Submission of the FDA New Drug Application.
- 2017: Completion of Ozempic clinical trials in May.
- 2017: In December, Ozempic gets FDA approval in the U.S. as an injectable semaglutide to enhance glycemic control in adults with type 2 diabetes.
- 2017: University of Leeds and Novo Nordisk find semaglutide effective in obesity treatment.
- 2018: Ozempic receives approval in the European Union, Japan, and Canada.
- 2019: The U.S. approves Novo Nordisk’s oral semaglutide, Rybelsus, for medical use.
- 2020: Rybelsus gains approval in the European Union.
- 2021: Wegovy, Novo Nordisk’s semaglutide injection for long-term weight management in adults, gets FDA approval.
- 2021: Over 10,000 semaglutide-related adverse events are reported to the FDA’s FAERS.
- 2023: FDA’s Drug Shortages list includes Ozempic and Wegovy.
- 2023: Filing of lawsuits against Novo Nordisk for patients who developed gastroparesis or stomach paralysis after using the drugs.
Contact our experienced Georgia legal team now for a free, no obligation case consultation. You may be entitled to substantial compensation for your injuries, harms and losses. Also, you owe no legal fees unless you win!
CALL US NOW – 678-242-7626 time to file a claim is limited, so you need to act now before your time to file a claim expires!
Request Free Consultation
"*" indicates required fields

